

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50264106 (CHEMBL3818875) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human sEH using MNPC as substrate by fluorescence-based assay | J Med Chem 61: 3541-3550 (2018) Article DOI: 10.1021/acs.jmedchem.7b01804 BindingDB Entry DOI: 10.7270/Q2ZP48KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50264106 (CHEMBL3818875) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF) Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase | J Med Chem 63: 6578-6599 (2020) Article DOI: 10.1021/acs.jmedchem.9b01782 BindingDB Entry DOI: 10.7270/Q2W95DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50264106 (CHEMBL3818875) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei... | J Med Chem 59: 6629-44 (2016) Article DOI: 10.1021/acs.jmedchem.5b01874 BindingDB Entry DOI: 10.7270/Q2N58QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50264106 (CHEMBL3818875) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei... | J Med Chem 59: 6629-44 (2016) Article DOI: 10.1021/acs.jmedchem.5b01874 BindingDB Entry DOI: 10.7270/Q2N58QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50595239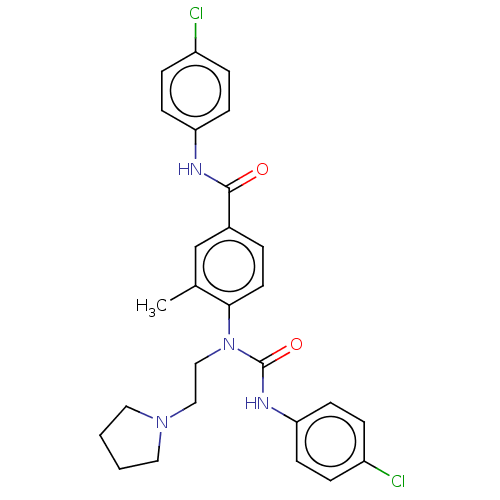 (CHEMBL5190894) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Bioorg Med Chem Lett 15: 3675-8 (2005) Article DOI: 10.1016/j.bmcl.2022.128805 BindingDB Entry DOI: 10.7270/Q2J67MXV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50556773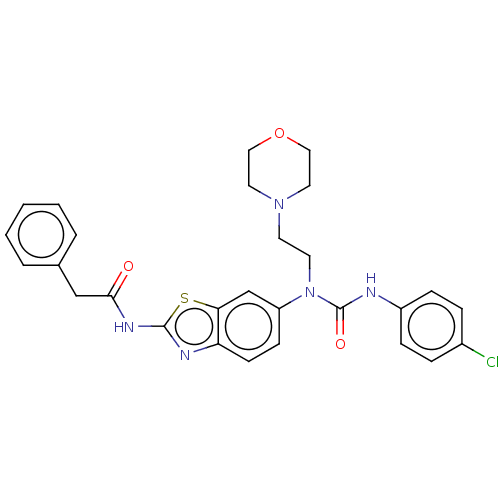 (CHEMBL4745610) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113028 BindingDB Entry DOI: 10.7270/Q208690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50302462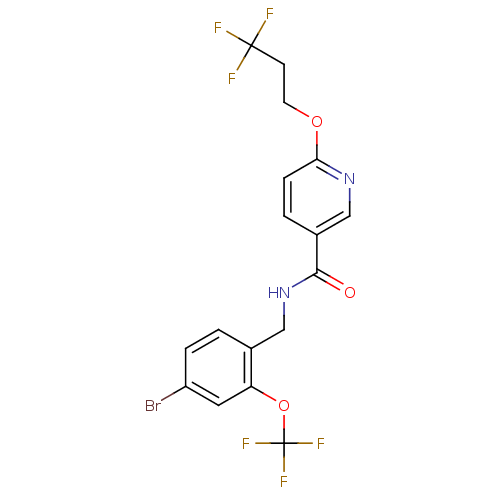 (CHEMBL566648 | N-(4-bromo-2-(trifluoromethoxy)benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of soluble EH in human HepG2 cells by cellular assay | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50556760 (CHEMBL4781218) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113028 BindingDB Entry DOI: 10.7270/Q208690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM342631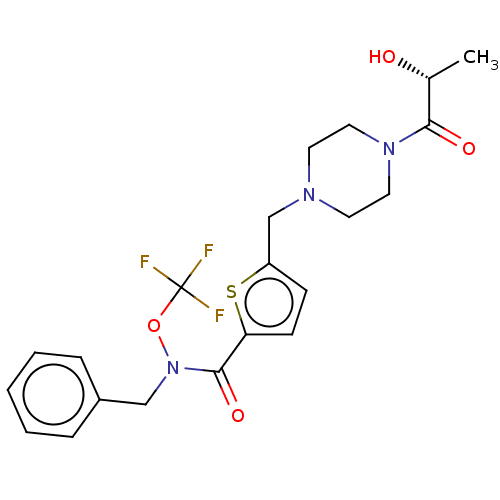 (5-[4-((R)-2-Hydroxy-propionyl)-piperazin-1-ylmethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Compounds were tested in a biochemical screening assay using recombinant sEH purified from Sf9 insect cells and an artificial substrate, (3-phenyl-ox... | US Patent US9776991 (2017) BindingDB Entry DOI: 10.7270/Q2V126ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445769 (CHEMBL3104615) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.125 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM342634 (5-[4-(Pyridine-3-carbonyl)-piperazin-1-ylmethyl]-t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.168 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Compounds were tested in a biochemical screening assay using recombinant sEH purified from Sf9 insect cells and an artificial substrate, (3-phenyl-ox... | US Patent US9776991 (2017) BindingDB Entry DOI: 10.7270/Q2V126ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441467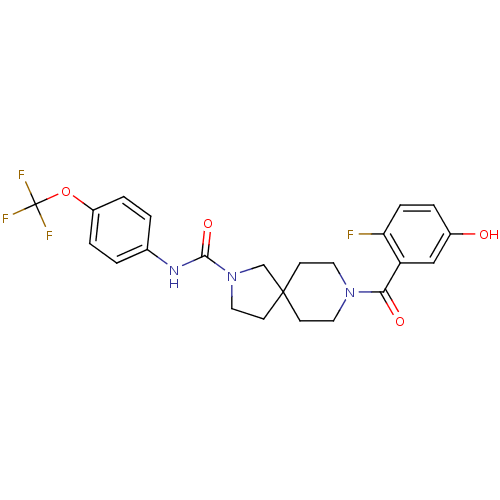 (CHEMBL2436573) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441481 (CHEMBL2436579) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441476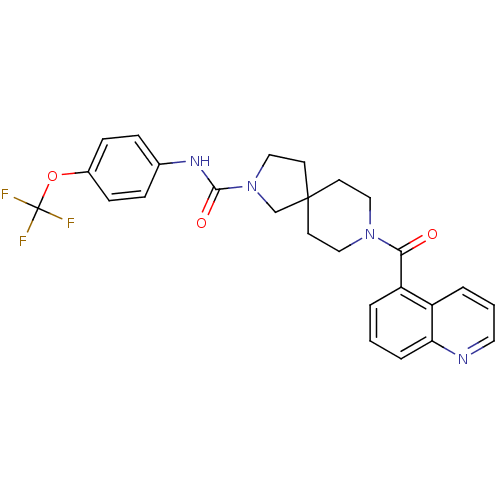 (CHEMBL2436575) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50556771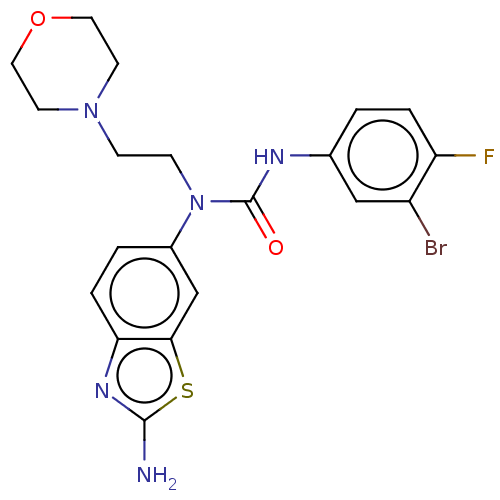 (CHEMBL4759847) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113028 BindingDB Entry DOI: 10.7270/Q208690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50595236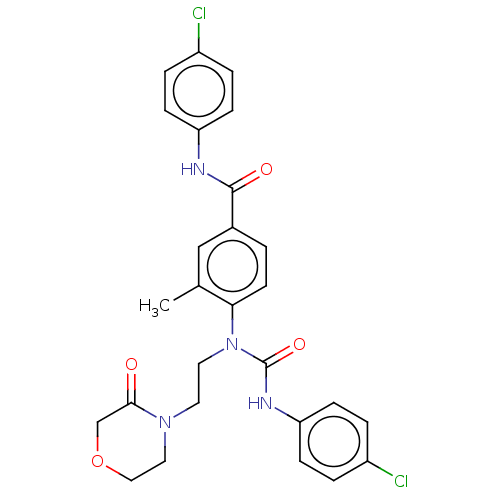 (CHEMBL5170467) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Bioorg Med Chem Lett 15: 3675-8 (2005) Article DOI: 10.1016/j.bmcl.2022.128805 BindingDB Entry DOI: 10.7270/Q2J67MXV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50595244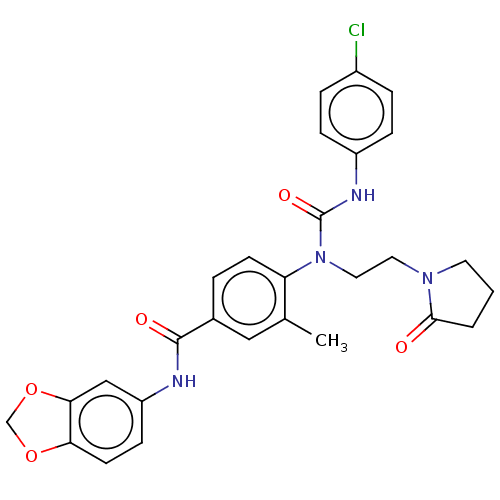 (CHEMBL5199526) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Bioorg Med Chem Lett 15: 3675-8 (2005) Article DOI: 10.1016/j.bmcl.2022.128805 BindingDB Entry DOI: 10.7270/Q2J67MXV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441483 (CHEMBL2436591) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441470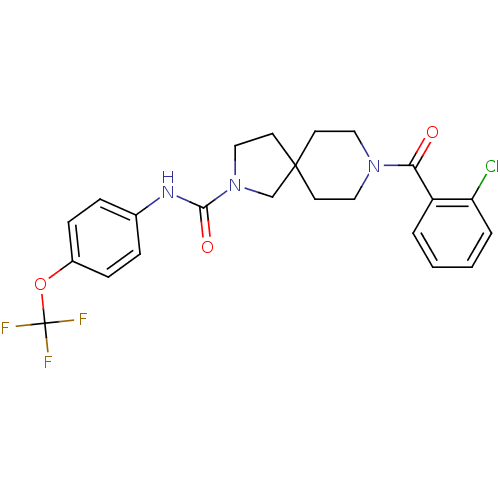 (CHEMBL2436574) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441469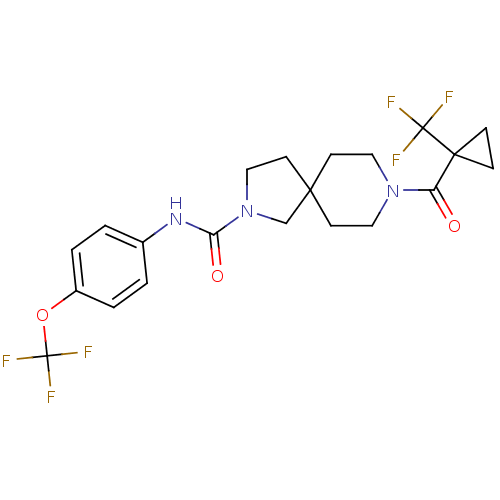 (CHEMBL2436586) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441474 (CHEMBL2436563) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50327850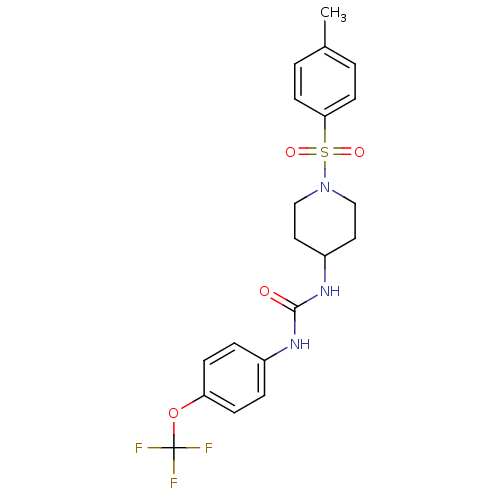 (1-(1-Tosylpiperidin-4-yl)-3-(4-(trifluoromethoxy)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of mouse recombinant soluble epoxide hydrolase by fluorescence assay | J Med Chem 53: 7067-75 (2010) Article DOI: 10.1021/jm100691c BindingDB Entry DOI: 10.7270/Q2GH9J6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351222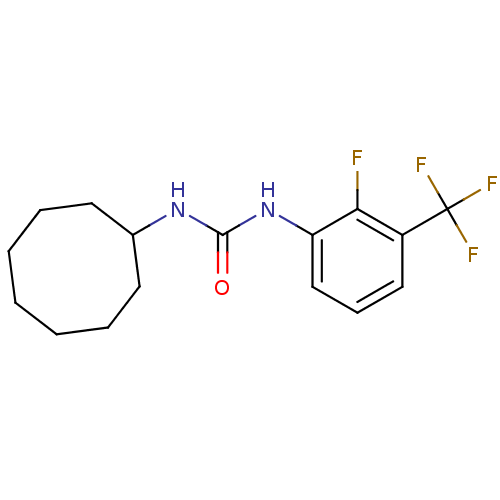 (CHEMBL1818408) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351223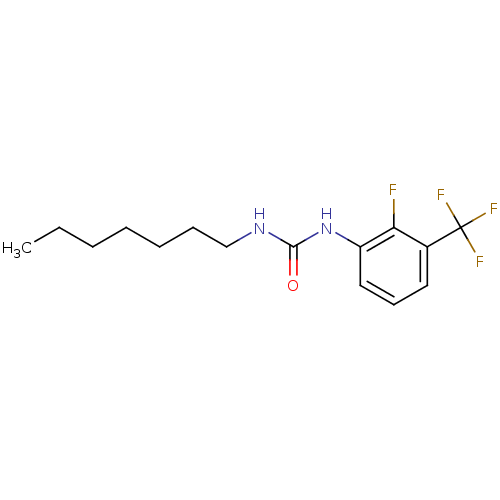 (CHEMBL1818407) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351226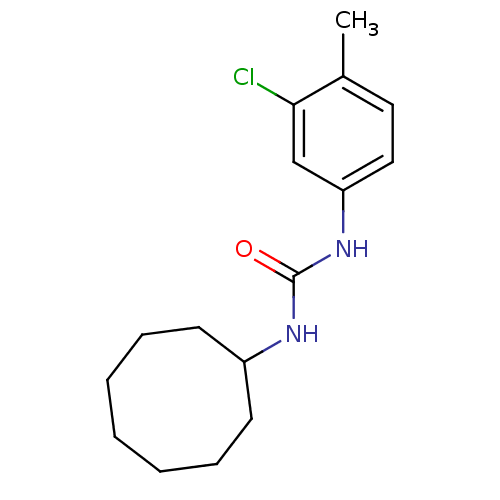 (CHEMBL1818404) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351228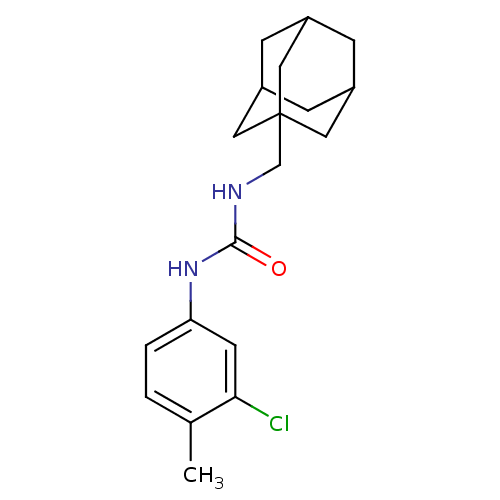 (CHEMBL1817677) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM53878 (4,5-dimethoxy-2-[2-(5-phenylthieno[2,3-d]pyrimidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351242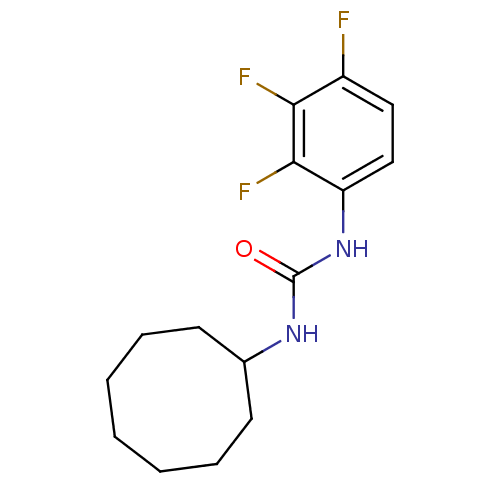 (CHEMBL1818390) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351243 (CHEMBL1818389) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351244 (CHEMBL1818388) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351245 (CHEMBL1818387) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351246 (CHEMBL1818386) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351247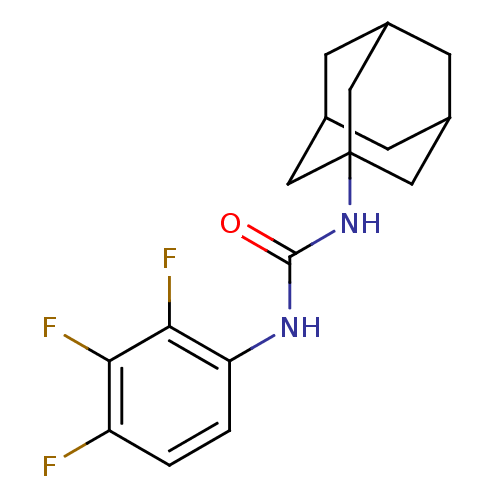 (CHEMBL1818385) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351248 (CHEMBL1818384) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate after 10 mins by fluorescent assay | Bioorg Med Chem 19: 5585-95 (2011) Article DOI: 10.1016/j.bmc.2011.07.034 BindingDB Entry DOI: 10.7270/Q2V69JZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50445752 (CHEMBL3104441) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... | Bioorg Med Chem Lett 24: 565-70 (2014) Article DOI: 10.1016/j.bmcl.2013.12.020 BindingDB Entry DOI: 10.7270/Q2V69M26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327844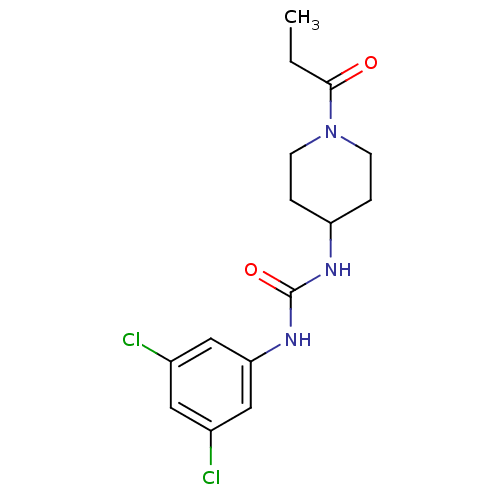 (1-(3,5-Dichlorophenyl)-3-(1-propionylpiperidin-4-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327845 (1-(4-Perfluoroisopropylphenyl)-3-(1-propionylpiper...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327846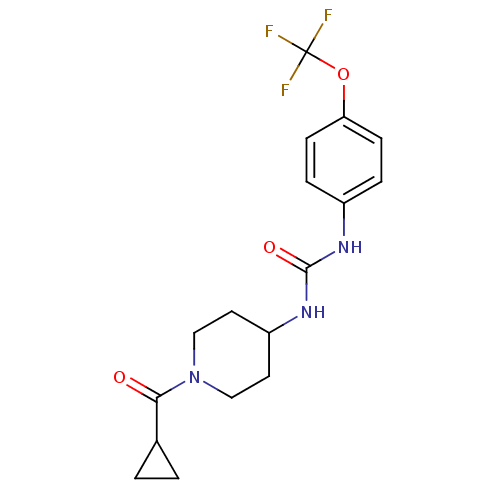 (1-(1-(Cyclopropanecarbonyl)piperidin-4-yl)-3-(4-(t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327847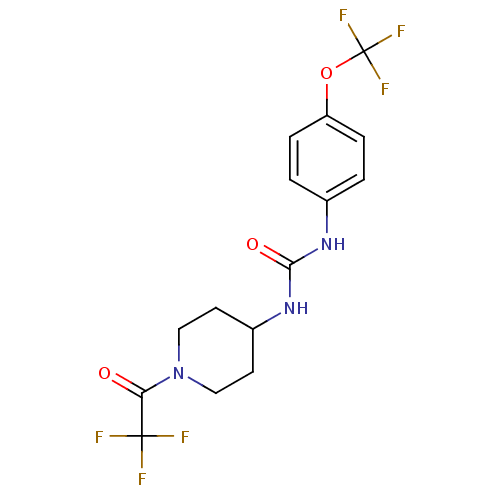 (1-(1-(Trifluoroacetyl)piperidin-4-yl)-3-(4-(triflu...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327848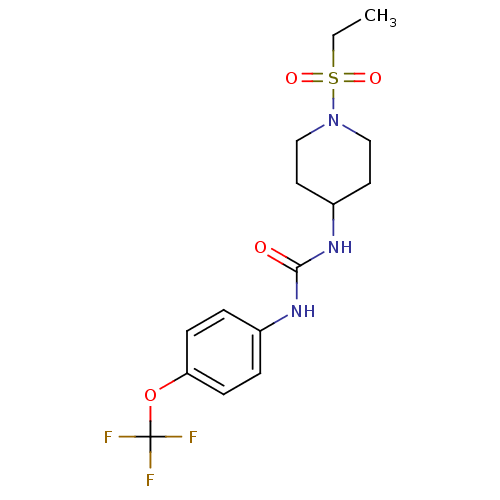 (1-(1-(Ethylsulfonyl)piperidin-4-yl)-3-(4-(trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327850 (1-(1-Tosylpiperidin-4-yl)-3-(4-(trifluoromethoxy)p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50327842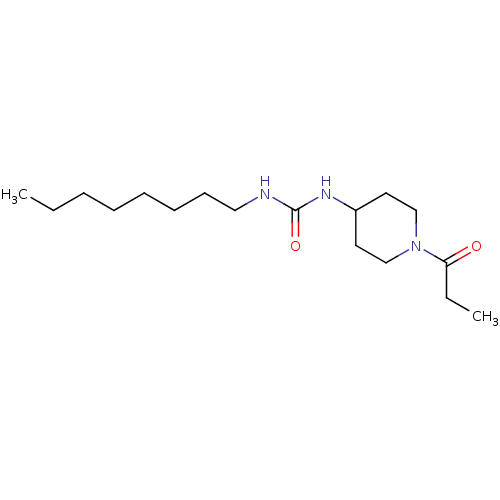 (1-Octyl-3-(1-propionylpiperidin-4-yl)urea | CHEMBL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50327843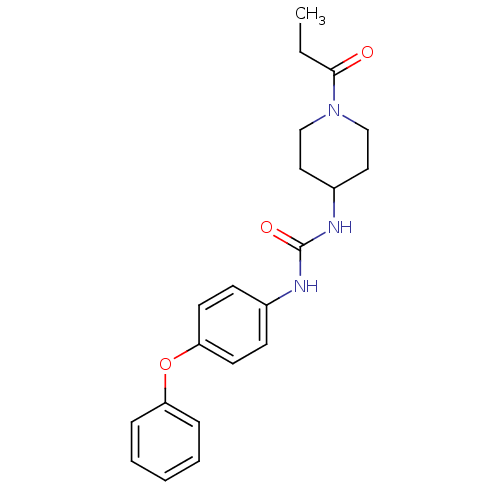 (1-(4-Phenoxyphenyl)-3-(1-propionylpiperidin-4-yl)u...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50327846 (1-(1-(Cyclopropanecarbonyl)piperidin-4-yl)-3-(4-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50327847 (1-(1-(Trifluoroacetyl)piperidin-4-yl)-3-(4-(triflu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50327849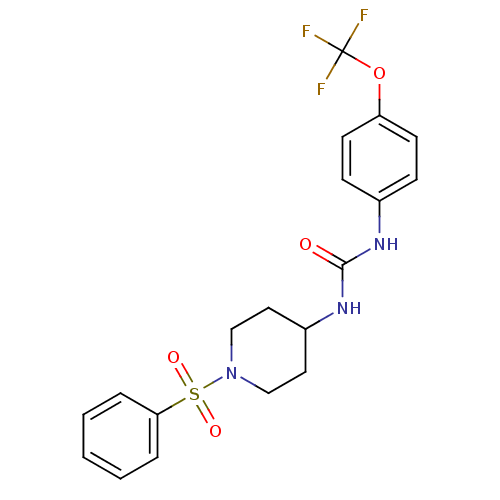 (1-(1-(Phenylsulfonyl)piperidin-4-yl)-3-(4-(trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50327850 (1-(1-Tosylpiperidin-4-yl)-3-(4-(trifluoromethoxy)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The Regents of the University of California US Patent | Assay Description IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-... | US Patent US9296693 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012011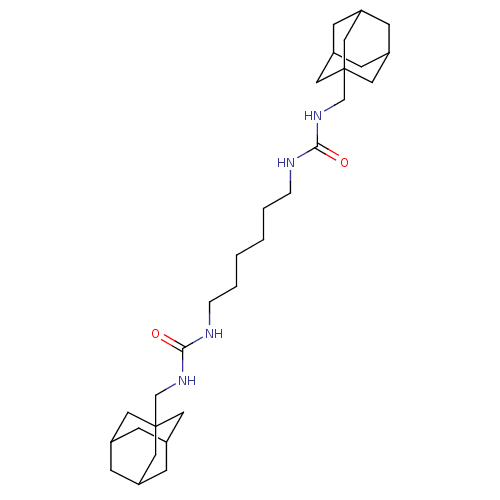 (CHEMBL3263289) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50577862 (CHEMBL4869434) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant sEH using PHOME as substrate measured after 10 mins by fluorescent based assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113678 BindingDB Entry DOI: 10.7270/Q2FF3X64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50577868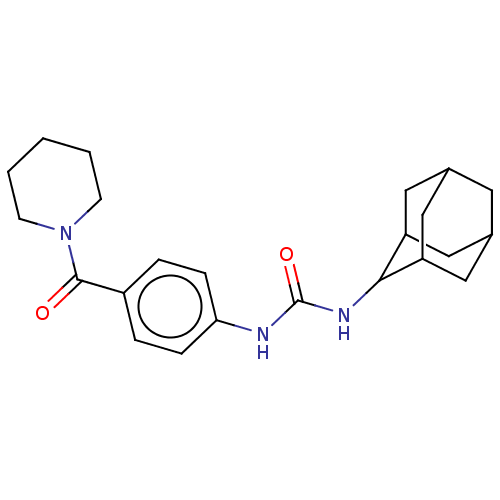 (CHEMBL4872738) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant sEH using PHOME as substrate measured after 10 mins by fluorescent based assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113678 BindingDB Entry DOI: 10.7270/Q2FF3X64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3573 total ) | Next | Last >> |